
CORNEA
THE OPPORTUNITY
A growing prevalence of diseases such as cataract, diabetic retinopathy, and glaucoma among others and high prevalence of eye diseases and vision impairment is likely to create lucrative growth prospects for the Corneal Surgery Devices market across the globe.
Corneal visual impairment causes scarring of the cornea and ultimately leads to functional vision loss. It is the 4th cause of blindness globally, after cataract, glaucoma and AMD, as indicated by World Health Organization (WHO).
For Oculus, the Company has entered into a Licensing and Collaboration Agreement with ACRO Biomedical Co., Ltd. to develop and market the ABCcolla® Collagen Ophthalmic Matrix (a bioregenerative cornea) product for lamellar keratoplasty in Australia and New Zealand with an option to collaborate in the Chinese market.
It is estimated that at least 5 million people in China suffer from blindness caused by cornea damage or a related disease. Each year, there are around 100,000 new cases however, only approximately 10,000 cornea surgical procedures are conducted principally through a lack of supplies of donated cornea. The opportunity to supply bioregenerative corneas regionally is compelling for Oculus.
Our Approach and Tech
The ABCcolla® Collagen Ophthalmic Matrix (“Bioregenrative Cornea”), originally invented and developed by ACRO Biomedical Co., Ltd.(“ACRO”), is an acellular cornea which is derived from the cornea of specific pathogen free (SPF) pig. The biocompatibility is excellent since the collagen proteins between human and pig are alike. To minimize the problem of host immune rejection, residual lipids, free proteins, cells, and nucleic acids are removed by a patented process with Supercritical CO2 (ScCO2) [YH Huang, et. al., Preparation of acellular scaffold for corneal tissue engineering by supercriticalcarbon dioxide extraction technology. Acta Biomaterialia, Vol. 58, 2017, pp. 238-243]. OBM is currently co-developed this Bioregenrative Cornea with ACRO.
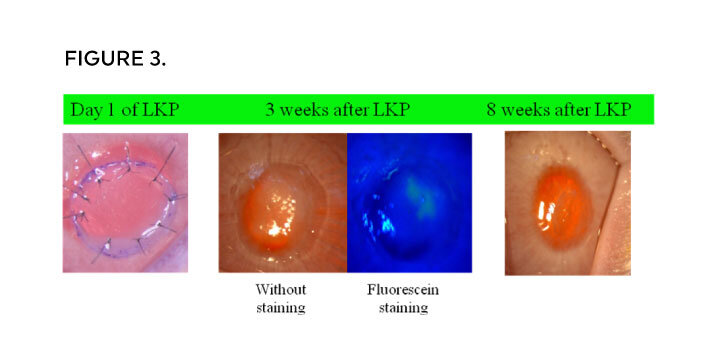
Results (FIGURE 3, from ACRO) from the LKP experiment of Bioregenrative Cornea on rabbit show positive results and excellent recovery after 8 weeks.
ACRO has been granted by Taiwan FDA in March 2020 to conduct the human clinical trials on Bioregenrative Cornea for LKP use. The clinical trials are currently underway in three major hospitals in Taiwan with GCP standards (https://clinicaltrials.gov/ct2/results?term=ACRO+Biomedical). The trials are for the Premarket Approval (PMA) for Class III medical devices. OBM is currently evaluating its opportunities to advance ABCcolla® Collagen Ophthalmic Matrix into clinical stages for PMA in its licensed territories.
