
THE OPPORTUNITY
An article published in the Journal of the British College of Ophthalmic Opticians stated that the global prevalence of dry eye disease (DED) was estimated at 11.59% in 2021.
Several factors, such as aging, decreasing supportive hormones, systemic inflammatory diseases, ocular surface diseases, or surgeries that affect the cholinergic nerves, which stimulate tear secretion, may be associated with the rising prevalence of dry eye-related diseases.
Importantly, our peptide not only has anti-inflammatory effect, but it also stimulates proliberation of cornea cells and increases tear production. This could result in alleviation of the symptoms and acceleration of cornea repair in DED patients.
Restasis (cyclosporine, Allergen) and Xiidra (lifitegrast, Novartis) are the two major approved drugs for DED with global annual sales in 2021 of US$1.29bn and US$468mln respectively.
Interestingly, Novartis bought the rights of Xiidra from Takeda of Japan in 2019 with a US$3.4bn upfront payment plus USD$1.9bn of potential milestone payments.
For Oculus, the drug development of OBM-17xx as a topical formulation for chronic use may provide significant opportunities in both potential revenue and corporate action.
Our Approach and Tech
- OBM-17xx is a synthetic peptide analogue and results from experiments show its inhibitory effects on inflammation and to markedly ameliorate clinical signs in the dry eye animal model by the topical application;
- OBM-17xx displays ffects of treatment and/or prophylaxis on corneal epithelial cells by countering inflammatory response induced by desiccating stress by placing mice in a controlled environment chamber . Corneal fluorescein staining (CFS) was performed to evaluate disease severity and data demonstrate that topical OBM-17xx treatment suppresses corneal inflammation and decreases the severity of DED (FIGURE 2).
- OBM-17xx has been successfully synthsised by the OBM’s CMO partner and its purity is 99.6% (by HPLC) has been obtained. Process for larger-scale manufacturing and a CMC, Chemistry, manufacturing and Controls, documentation can be built and prepared when required.
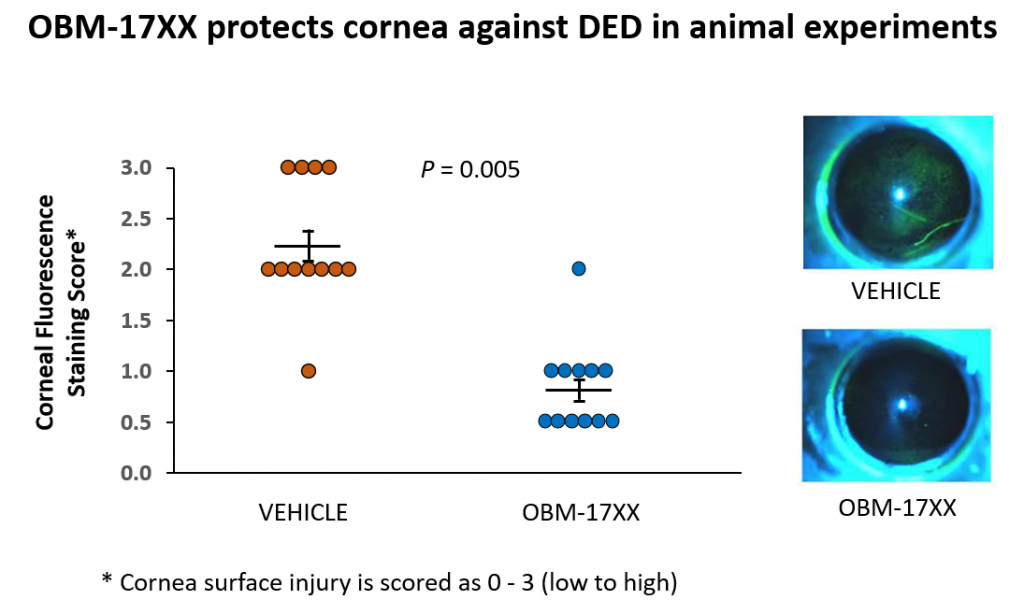
Mice were housed at CEC for 10 days to induce dry eye and topically treated with either vehicle (1% CMC) or OBM-17xx three times per day. After 10 days, corneal epithelial defect was assayed by fluorescein dye staining. Each eye was scored on a scale 0-3 depending on the area stained by fluorescein [Horwath-Winter J, et al., Br J Ophthalmol. 2013;97:466–470]. Results revealed the staining scores were significantly reduced by the OBM-17xx treated eyes, compared to the vehicle-treated eyes (P=0.005).
